Empirical Formula of Benzene
The structural formula of a chemical compound is a graphic representation of the molecular structure determined by structural chemistry methods showing how the atoms are possibly arranged in the real three-dimensional spaceThe chemical bonding within the molecule is also shown either explicitly or implicitly. Benzene a 352 toluene a 613 ethy lbenz ene a 1065 o-xy lene a 1292 m-xy lene a 1133 p-xy lene a 1104 cumene b 1861 p-ter t-bu ty lt oluene b 2145 -methyl sty rene b 1999 -methyl sty rene b 2082 styrene b 1833 a Separation achieved using a 30-m Stabilwax fused silica capillary colum.

The Various Representations Of Benzene Organic Chemistry Benzene Chemistry Lessons
Eilhardt Mitscherlich synthesized benzene in 1834 and showed it to have a molecular formula of C 6 H 6Many other compounds with similar properties to benzene were discovered in the 1800s all.
. The structural formula is shown below. First select Enthalpy Formula option. All solvents exist on a continuum of polarity.
Its molecular weight is 24124. The chemical name of Methocarbamol is 3-2-methoxyphenoxy-12-propanediol 1-carbamate and has the empirical formula C11H15NO5. In ketones the carbonyl group has 2 hydrocarbon groups attached to it.
In pentanone the carbonyl group could be in the middle of the chain or next to the. For the spectrum on the right a solution of 0249 mg of the unsaturated aldehyde in 95 ethanol 142 10-5 M was placed in a 1 cm cuvette for measurement. These can be either the ones containing benzene rings or alkyl groups.
Draw a structural formula for the substitution product of the reaction shown below. Separate multiple products using the sign from the drop-down menu. With the chart below the empirical formula of the compound can be used to determine solubility by cross referencing the cations top row with anions first column.
Its molecular weight is 24124. What is the molar mass of the compound. Benzene was first isolated by Michael Faraday in 1825 from the whale oil used in gaslights.
The structural formula is shown below. He also determined that it had an empirical formula of CH. Ebullioscopic constant for benzene.
The chemical name of methocarbamol is 3-2-methoxyphenoxy -12propanediol 1-carbamate and has the empirical formula C 11 H 15 NO 5. Using the above formula ε 36600 for the 395 nm peak and 14000 for the 255 nm peak. The molecular formula of benzene is C₆H₆.
If more than one stereoisomer of product is formed draw both. The complete list of chapters and subtopics of the Class 11 NCERT textbook has been provided above. B Separation achieved using a 30-m Rtx-35 fuse d.
Download PDF Solvent Polarity. Calculate the molecular weight of the compound. Stoichiometry Stoichiometric Calculations The coefficients in the balanced equation give the ratio of moles of reactants and products.
Thus it has six carbon atoms and it needs 8 more hydrogen atoms in order to be classified as saturated. For less common compounds you can consult a periodic table while using the solubility guidelines listed above. Its molecular weight is 24124.
253 Cm and Boiling Point of pure benzene. A 115 gmmol b 145 gmmol c 120 gmmol d 100 gmmol. Examples of other chemical formulae for butane are the empirical formula C 2 H 5 the molecular formula C 4 H 10 and the condensed or semi-structural formula CH 3 CH 2 CH 2 CH 3.
The enthalpy calculator calculates. Ketone does not have a hydrogen atom attached to the carbonyl group. These are the subscripts for the empirical formula.
Then put values against each parameter in tool and calculate. F CH3 Na OČCH3 CHCO2H Use the wedgehash bond tools to indicate stereochemistry where it exists. This page can help provide context and structure for learning the fundamental principles covered under the Class 11 CBSE chemistry syllabus thereby empowering the students to develop from simple to relatively complex concepts in a systematic manner.
In chemistry a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule using chemical element. A solution of 100 g of a nonvolatile non-dissociating compound dissolved in 0200 kg of benzene boils at 812 C. Propane is generally written as CH3COCH3.
Solution for 1365 g of an unknown compound decreased the freezing point of 346 g of benzene Kf 512 C m by 129 C. Therefore the degree of unsaturation of benzene is 4 which gives one ring and three double bonds. The overall variation in heat and shows you with the step-by-step calculation corresponding to the given values and.
After doing so select either Internal energy volume or Change in internal energy volume. C 7H 7NO 2 H2N O O-Stoichiometry Elemental Analyses Compounds containing other elements are analyzed using methods analogous to those used for C H and O. Unlike other chemical formula types which have a limited.
What Is The Empirical Formula Of Benzene Quora

C6h6 Lewis Structure Benzene Lewis Chemical Formula Home Decor Decals
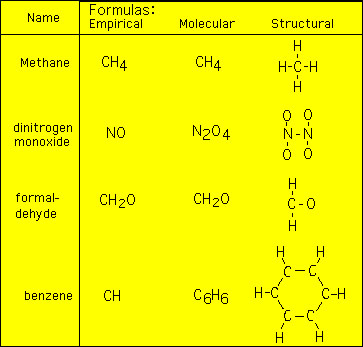
Quantitative Chemistry Molecular Formulas

The Empirical Formula Of Benzene And Acetylene Is Are Youtube
0 Response to "Empirical Formula of Benzene"
Post a Comment